Abstract
Background:FLT3 is a member of the class III receptor tyrosine kinase family and constitutively activating mutations of FLT3 are found in ∼30% of adult patients with acute myeloid leukemia (AML). Clinically, FLT3-ITD mutations are associated with earlier time to relapse and poorer overall survival (OS). FLT3 inhibitors are divided into first generation multi-kinase inhibitors (such as sorafenib, lestaurtinib, midostaurin) and next generation inhibitors (such as quizartinib, crenolanib, gilteritinib) or based on their specificity of FLT3 inhibition [type I inhibitors (midostaurin, gilteritinib) inhibit FLT3 ITD and TKD mutations whereas type II inhibitors (sorafenib, quizartinib) have only FLT3 ITD activity]. Though FLT3 inhibition has showed marginal survival benefit in the front line setting for AML, some of the benefits may be offset by cardiovascular toxicities such as cardiomyopathies, QT prolongation and arrythmias. Therefore, outcomes and cardiac safety profile of FLT3 inhibitors in AML at our institution was investigated in the real-life scenario.
Methods: In this retrospective study, we included all FLT3MTAML patient with ECOG status of ≤ 2 at our institute who either received FLT3 inhibitors (1) at diagnosis, (2) in a study after being refractory to ≥1 treatment, or (3) relapsing after achieving prior remission. We analyzed the safety and tolerability of type I and type II FLT3 inhibitors in terms of dose-limiting toxicities (DLT) and treatment-emergent adverse events (TEAE) from a cardiac standpoint. Baseline 2D Echocardiograms, EKGs pre- and post-treatment, metabolic profile through each hospitalization, major cardiac events in correlation to prior cardiac history and electrolyte abnormalities were included in the final analysis.
Results: From June 2016 to April 2021, 63 FLT3MT AML patients were treated at our institution, 41 (65%) with primary AML and 22 with secondary AML (35%). 52 patients (83%) had ITD and 11 (17%) had FLT3 TKD mutations. Furthermore, 29 patients (56%) with FLT3 ITD and 3 (27%) with FLT3 TKD were treated with FLT3 inhibitors. Of the patients who received FLT3 inhibitors, 21 (66%) received type-2, and 11 (34%) got type I FLT3 inhibitors. 17 patients received FLT3 inhibitors as mono-therapy, and 15 in combination with chemotherapy. Median age of patients on FLT3 inhibitor treatment was 77 years (range 40- 83), and median duration of treatment was 96 days (range 45-180). CRi and CRp were seen as the best response in 12 (38%) and 5 (17%) of the 32 FLT3MT patients respectively, and the median time to CRi/CRp was 33 days (range 22 to 36). Time to relapse in patients with CRi/CRp was 4.5 months (range 2 to 7.5 months). Median overall survival of FLT3 ITD patients treated with type II inhibitors was 18 months, versus 14.5 months for type I inhibitors (p = 0.782), and 5.6 months in patients who did not receive FLT3 inhibitor therapy.
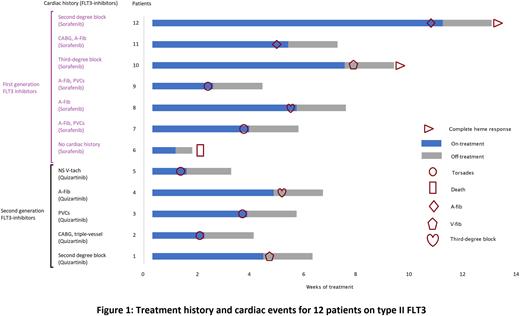
The most common non-cardiac treatment emergent adverse events (TEAEs) in patients on FLT3 inhibitors were fatigue, muscle weakness, anemia, and decreased appetite. In the total FLT3MT AML cohort, cardiac adverse events were observed in 16 patients (16/63), 12 of which were on FLT3 inhibitors and 4 were not on FLT3 inhibitors (p = 0.0006). All cardiac TEAEs with FLT3 inhibitor therapy were observed only with type II inhibitors (p=0.0018). Notably, 12 (12/32) patients were classified to have serious cardiac TEAEs, and all of them were receiving type II FLT3-inhibitors. Serious cardiac TEAEs included Torsades in 5 (41%) patients, recurrent ventricular fibrillation in 2 (17%), third-degree AV block necessitating emergent pacemaker in 2 (17%), permanent atrial fibrillation in 2 (17%), and asystole culminating in death in 1 patient (8%) [Fig.1]. 12 patients who developed cardiac TEAEs with FLT3 inhibitors had hyperkalemia, 5 had a preceding history of atrial fibrillation and PVC, suggesting a decreased threshold of development of cardiac TEAEs in the presence of known risk factors.
Conclusions: Type II FLT3 TKIs had worse cardiac safety profile in our cohort and an increased risk of cardiac toxicity, particularly in patients with prior reduced ejection fraction or A-fib. A careful cardiac history and management of predisposing conditions to serious cardiac events could help improve drug tolerance, morbidity, and mortality. Further studies are required to elucidate the precise mechanisms of increased incidence of cardiac arrythmias and ways to mitigate them.
Disclosures
Balasubramanian:Kura Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal